|
A general approach to crystalline and monomodal pore size mesoporous materials |
|
|
|
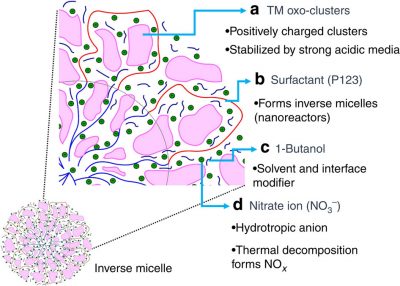
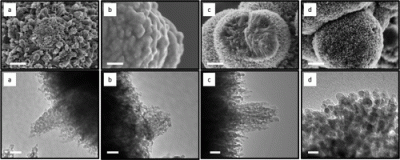
Mesoporous oxides attract a great deal of interest in many fields, including energy, catalysis and separation, because of their tunable structural properties such as surface area, pore volume and size, and nanocrystalline walls. Here we report thermally stable, crystalline, thermally controlled monomodal pore size mesoporous materials. Generation of such materials involves the use of inverse micelles, elimination of solvent effects, minimizing the effect of water content and controlling the condensation of inorganic frameworks by NOx decomposition. Nanosize particles are formed in inverse micelles and are randomly packed to a mesoporous structure. The mesopores are created by interconnected intraparticle voids and can be tuned from 1.2 to 25 nm by controlling the nanoparticle size. Such phenomena allow the preparation of multiple phases of the same metal oxide and syntheses of materials having compositions throughout much of the periodic table, with different structures and thermal stabilities as high as 800 °C.
|
|
[Nature Communications, 4 doi:10.1038/ncomms3952] |
|
|
…………………………………………………………………………………………………………………………………………………………………… |
|
|
Mesoporous Co3O4 with Controlled Porosity: Inverse Micelle Synthesis and High-Performance Catalytic CO Oxidation at −60 °C |
|
|
|
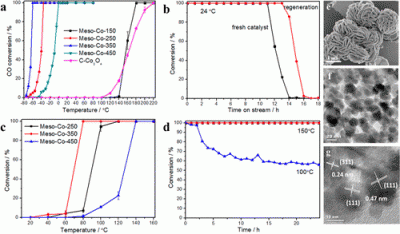
Crystalline mesoporous cobalt oxides with improved catalytic activity in CO oxidation were synthesized using an inverse surfactant micelle method. The prepared materials are monodispersed nanoparticle aggregates, and the mesopores are formed by connected intraparticle voids. Powder X-ray diffraction (PXRD), N2 sorption, field emission scanning electron microscope (FE-SEM) and high-resolution transmission electron microscopy (HR-TEM) revealed that both pore and nanoparticle sizes are enlarged with increasing thermal treatment temperatures (150–450 °C). Mesoporous cobalt oxide calcined at 350 °C exhibited the best oxidation activity and can achieve complete oxidization (100% conversion) of CO to CO2 at −60 °C under normal conditions (∼3–10 ppm of H2O) and at 80 °C under moisture rich conditions (∼3% H2O). The commercial Co3O4 reached 100% conversion at 220 °C under normal conditions. X-ray photoelectron spectroscopy (XPS), O2-temperature-programmed desorption (O2-TPD), H2-temperature-programmed reduction (H2-TPR), CO-TPD, and N2 sorption analyses indicated that the surface oxygen vacancy and large surface area promoted the lattice oxygen mobility of the catalysts and further enhanced their catalytic performance. The catalysts were deactivated by accumulation of water and formation of carbonates, but their activities can be easily restored by expelling water and carbonates at moderate temperature (200 °C). |
|
[Chem. Mater. 2014, 26 (15), 4629–4639] |
|
|
…………………………………………………………………………………………………………………………………………………………………… |
|
|
Ion induced promotion of activity enhancement of mesoporous manganese oxides for aerobic oxidation reactions |
|
|
|
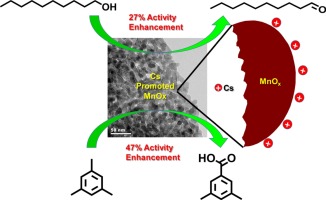
Inverse micelle templated mesoporous manganese oxide (University of Connecticut mesoporous material, UCT-1) and ion promoted mesoporous manganese oxides (UCT-18-X, X = Mg2+, Ca2+, K+, Na+ and Cs+) having trace amount of alkali metal ions as promoters with tunable porosity and crystallinity were synthesized. The synthesis was based on the use of inverse micelles as soft templates and unique NOx chemistry as established for recently discovered UCT materials. The materials were tested for selective aerobic alcohol oxidation and the catalytic activity followed the order of UCT-1 < UCT-18-Mg < UCT-18-Ca < UCT-18-K < UCT-18-Na < UCT-18-Cs. The catalytic activity was correlated to the promoter ion induced increase in basicity of the materials. The retention of an amorphous structure, low reducibility, and the effects of lattice oxygen are other key factors responsible for the enhanced catalytic activity. The UCT-18-Cs catalyst was found to oxidize various alcohols to corresponding aldehydes and ketones selectively (100% selectivity) with very high conversion (as high as 100%). The UCT-18-Cs also exhibited solvent free green oxidation of 1.3.5-trimethylbenzene to yield 3,5-dimethylbenzoic acid and (3,5-dimethylphenyl)methyl ester with >90% conversion and 90% selectivity for the acid and 9% selectivity for the ester. |
|
[Applied Catalysis B: Environmental, 2015, 165, 731-741] |
|
|
…………………………………………………………………………………………………………………………………………………………………… |
|
|
Robust Mesoporous Manganese Oxide Catalysts for Water Oxidation |
|
|
|
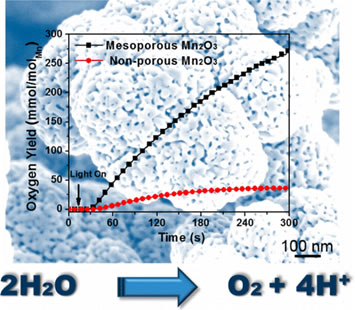
Inspired by the natural oxygen evolution reaction of Photosystem II, the earth-abundant and inexpensive manganese oxides (MnOx) have been recognized for their great potential as highly efficient and robust materials for water oxidation reaction (WORs). To date, most of the heterogeneous, synthesized MnOx catalysts still exhibit lower activities for WORs, in comparison to RuO2 and IrO2. Herein, we report a single-step and scalable synthesis method for mesoporous MnOx materials that is developed through a soft-templated method. This method allowed precise control of Mn3+-rich Mn2O3 structure as well as pore sizes and crystallinity of these mesoporous MnOx. These catalysts were investigated for both photochemical and electrochemical water oxidation, and they presented a superior activity for water oxidation. The highest turnover frequency of 1.05 × 10−3 s −1 was obtained, which is comparable with those for precious metal oxide based catalysts (RuO2 and IrO2). Our results illustrate a guideline to the design and synthesis of inexpensive and highly active heterogeneous catalysts for water oxidation. |
|
[ACS Catalysis, 2015, 5(3), 1693-1699] |
|
|
…………………………………………………………………………………………………………………………………………………………………… |
|
|
Synthesis of Mesoporous Iron Oxides by an Inverse Micelle Method and Their Application in the Degradation of Orange II under Visible Light at Neutral pH |
|
|
|
Mesoporous iron oxides (2-line ferrihydrite, α-Fe2O3, γ-Fe2O3, and Fe3O4) are successfully synthesized by modifying the reaction temperatures and calcination atmospheres of the sol–gel-based inverse micelle method. Different characterization techniques, such as PXRD, N2sorption, SEM, HRTEM, Raman spectroscopy, and XANES, are performed to determine the properties of the catalysts. Larger pore sizes can be obtained in mesoporous γ-Fe2O3 and Fe3O4 compared with mesoporous 2-line ferrihydrite and α-Fe2O3. The catalytic performance of mesoporous iron oxides are examined as Fenton catalysts in orange II degradation in the presence of oxidant H2O2 at neutral pH under visible light. Adsorption capacities of mesoporous iron oxides on orange II are greater than that of commercial Fe2O3. The greatest adsorption capacity is found to be 49.3 mg/g with mesoporous 2-line ferrihydrite. In addition, the degradation efficiency of orange II is found to be markedly improved by mesoporous iron oxides compared with the commercial catalyst. In the best case scenario, 2-line ferrihydrite shows the highest degradation rate constant (0.0258 min–1) among all the catalysts tested. The excellent performance of 2-line ferrihydrite is mainly attributed to the larger surface area but also related to surface hydroxyl groups, acidic products, and possible additional adsorption sites. The recyclability of mesoporous 2-line ferrihydrite catalyst can be achieved up to 3 times without performance decay. At last, a discussion regarding the possible mechanisms of degradation of orange II over mesoporous 2-line ferrihydrite is proposed, based on the previous literature work and the observed reaction intermediates monitored by ESI/MS in this study. |
|
[The Journal of Physical Chemistry C. 2015] |
|
|
…………………………………………………………………………………………………………………………………………………………………… |
|
|
High-Performance Catalytic CH4 Oxidation at Low Temperatures: Inverse Micelle Synthesis of Amorphous Mesoporous Manganese Oxides and Mild Transformation to K2–x Mn8O16 and ϵ-MnO2 |
|
|
|
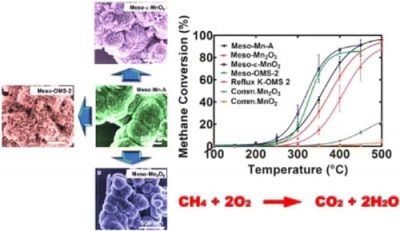
Catalytic combustion of methane at low temperature under lean conditions was investigated over mesoporous amorphous manganese oxide (Meso-Mn-A), Mn2O3 (Meso-Mn2O3), MnO2(epsilon phase) (Meso-ϵ-MnO2), and octahedral molecular sieves MnO2 (Meso-OMS-2) synthesized using an inverse surfactant micelle method. The prepared materials are monodispersed nanoparticle aggregates, and the mesopores are formed by connected interparticle voids. All the mesoporous manganese oxides proved to be significantly active compared to nonporous, similar phase materials. However, among the tested materials Meso-Mn-A showed the lowest light-off temperature of 229 °C, but Meso-OMS-2 showed the highest conversion (90%) at the lowest temperature of 373 °C. Despite the low light-off temperatures of mesoporous materials, even nonporous K-OMS-2 (cryptomelane) showed 90% conversion at 403 °C illustrating not only the effect of mesopore size but also the oxidation state of manganese and the structure of the catalyst having effects on the activity of manganese oxides. X-ray photoelectron spectroscopy (XPS), H2-temperature-programmed reduction (H2-TPR), and N2 sorption analysis indicated that the oxidation states of catalysts, surface oxygen vacancies, and large surface areas promoted the lattice oxygen mobility of the catalysts. Thus, activities of the catalysts were correlated to the oxidation states, the lattice oxygen mobility, and the reducibility of the catalysts. The apparent activation energy of methane oxidation calculated based on a pseudo-first-order kinetics ranged from 70.5 to 107.2 kJ mol–1 for the manganese oxides, and the values are comparable with catalysts containing precious metals.
|
|
[The Journal of Physical Chemistry C, 2015, 119(3), 1473-1482] |
|