|
Heteroepitaxial Growth of Nanoscale Oxide Shell-Fiber Superstructures by Mild Hydrothermal Processes (Cover story) |
|
|
|
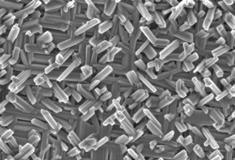
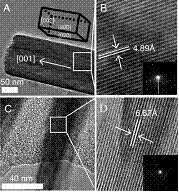
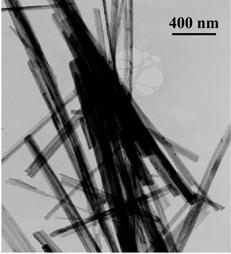
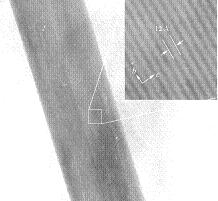
The growth of nano-sized crystalline materials with orientational and morphological control is crucial for considerable applications, however, even when this can be achieved, the preparation techniques required are usually complex and specific to a single system. We demonstrate here an inexpensive and scaleable hydrothermal route to produce heteroepitaxial rutile TiO2 nanoscale shell-fiber superstructures (NSFS) on cryptomelane MnO2 (OMS-2) substrates. The application of this procedure to thin film cryptomelane MnO2 substrates resulted in uniform TiO2 nanofiber arrays over large areas indicating the potential of this approach for application in sensors and energy-harvesting devices. The cryptomelane MnO2 substrate has also been removed from the superstructures chemically to give hollow nano-structures. The successful extension of the work to the production of epitaxial rutile SnO 2 NSFS indicates that this may be the basis for a much more general approach for the growth of metal oxide nanostructures. |
|
[ Small, 2010, 6 (9), 988-992 ] |
|
|
…………………………………………………………………………………………………………………………………………………………………… |
|
|
Nanostructured arrays of semiconducting octahedral molecular sieves by pulsed-laser deposition |
|
|
|
Cryptomelane-type manganese oxide (OMS-2) was widely used to explore the semiconducting and catalytic properties of molecular sieves with mixed-valent frameworks. Selective synthesis of patterned thin films of OMS-2 with hierarchical nanostructures and oriented crystals is challenging owing to difficulties in preserving the mixed valence, porosity and cryst. phase. Here, the authors report that pulsed-laser ablation of OMS-2 in an oxygen-rich medium produces a three-dimensional nanostructured array of parallel and inclined OMS-2 fibers on bare substrates of (001) single-crystal strontium titanate. Both parallel and inclined OMS-2 fibers elongate along the [001]OMS-2 direction. The parallel fibers interact strongly with the substrate and grow epitaxially along .ltbbrac.110.rtbbrac.STO with lattice misfits of <4%, whereas the inclined fibers are oriented with (301) parallel to the substrate surface. The spontaneous orientation of the cryst. OMS-2 domains over the STO surface opens up a new avenue in lattice-engineered synthesis of multilayer materials.
|
|
[ Nature Materials 2010, 9, (1), 54-59 ] |
|
|
…………………………………………………………………………………………………………………………………………………………………… |
|
|
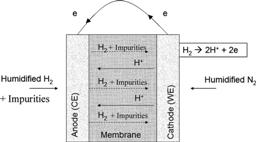
Influence of formic acid impurity on proton exchange membrane fuel cell performance |
|
|
|
The effect of trace amts. of formic acid (HCOOH) on proton exchange membrane fuel cell (PEMFC) performance is reported. Exptl. approaches including long term stability test (100 h), cyclic voltammetry (CV) scans and electrochem. impedance spectroscopy (EIS) were used to evaluate and characterize the contamination. The results show that trace amts. of HCOOH can cause degrdn. in fuel cell performance and significantly contaminate the electrodes. Furthermore, fully recovery from the contamination could not be achieved only by reverting to pure hydrogen while operating the fuel cell. Mechanisms of contamination of the electrodes and performance degrdn. of the PEMFC are also postulated.
|
|
[ Journal of the Electrochemical Society 2010, 157, (3), B409-B414 ] |
|
|
…………………………………………………………………………………………………………………………………………………………………… |
|
|
Synthesis of mordenite aggregates of nanometer-sized crystallites |
|
|
A high SiO2/Al2O3 ratio (SAR 15) mordenite was prepd. in a very short reaction time of only 12 h. The syntheses were accomplished in the presence of alc. additives using both microwave and conventional heating methods. Mordenite crystn. was greatly enhanced using a combination of alc. additives and conventional heating. Mordenite nucleation was also increased without using alcs. when microwave heating was employed, but the alcs. further accelerated the nucleation process. The different heating techniques affected the morphol.; microwave heating produced crystallites of 40 nm, while the conventional hydrothermal method formed larger size crystallites of 88 nm. We controlled the size and shape of the mordenite crystals because they have important implications in hydrocarbon conversion and sepn. processes. SEM micrographs of mordenite showed jellyfish, acicular, flower, and wheat grain like structures. |
|
|
[ Science of Advanced Materials 2009, 1, (1), 31-37 ] |
|
|
…………………………………………………………………………………………………………………………………………………………………… |
|
|
ZnO with Different Morphologies Synthesized by Solvothermal Methods for Enhanced Photocatalytic Activity |
|
|
|
ZnO materials with a range of different morphologies have been synthesized via a simple solvothermal method with different solvents. Zinc acetylacetonate was used as the zinc source in such solvothermal syntheses for the first time. XRD data showed that single-phase ZnO with the wurtzite crystal structure was obtained for all the solvents used. FE-SEM imaging showed that ZnO with cauliflower-like, truncated hexagonal conical, tubular and rodlike, hourglass-like, nanorods, and spherical shapes were produced when THF, decane, water, toluene, ethanol, and acetone were used as the solvent, respectively. TheTEMdata showed that the crystalline ZnO had different growth habits in the different solvents. The optical properties of the as-prepared ZnO materials were investigated byUV-vis absorption and room temperature photoluminescence. Photodegradation of phenol was used as a model reaction to test the photocatalytic activity of the ZnO samples. ZnO samples with different morphologies and crystal growth habits exhibited different activities to phenol degradation. The ZnO material prepared usingTHFas the solvent showed a nine-times enhancement of the kinetic rate constants over commercial ZnO (0.1496 min-1 vs 0.0182 min-1). The influence of the solvents on the morphology of ZnO samples and the effect of the morphologies on the photocatalytic activity are discussed.
|
|
[ Chemistry of. Materials (2009) 21, (13), 2875-2885 ] |
|
|
…………………………………………………………………………………………………………………………………………………………………… |
|
|
Urchin-like CuO Synthesized by a Facile Reflux Method with Efficient Olefin Epoxidation Catalytic Performance |
|
|
|
CuO is an important transition metal oxide with a narrow bandgap (Eg ) 1.2 eV). CuO has been used as a catalyst, a gas sensor, in anode materials for Li ion batteries. CuO has also been used to prepare high temperature superconductors and magnetoresistance materials. In this paper, CuO with urchin-like morphologies has been synthesized via a simple reflux method. The reflux method has advantages over other solution-based techniques, such as ease of operation, safety, and high yield (95%). XRD results showed pure tenorite CuO was produced. FE-SEM exhibited an urchin-like morphology of CuO, which is composed of aggregates of nanosized strips. HR-TEM showed that the strips were single crystals with the lattice fringe of 2.3 Å, which corresponds to (111). DSC and TGA results suggested that as-synthesized CuO had high thermal stability. Time-dependent experiments were conducted to illustrate the evolution of the urchin-like morphology and crystal phase formation of CuO. The effects of copper sources and precipitators on the phase and morphology of the products were studied. As-synthesized CuO showed much better catalytic performance, increased yield (from 64.3% to 89.5%) for olefin epoxidation than commercial CuO and CuO prepared by thermal decomposition of copper hydroxide.
|
|
[ Chemistry of. Materials (2009) 21, (7), 1253-1259 ] |
|
|
…………………………………………………………………………………………………………………………………………………………………… |
|
|
Role of Ti-O bonds in phase transitions of TiO2 |
|
|
|
A phase transition was achieved from TiO2 particles to titanate nanotubes by the breakage of Ti-O bonds without using expensive precursors, high temperatures, high chemical concentrations of alkaline solutions, and long synthesis times. The controlled preparation of one-dimensional nanostructures of titanates (H2Ti4O9 3 nH2O) has been conducted at a very low concentration of alkaline solution (1MNaOH), and in a very short time (12 h) using TiO2 anatase and TiO2 P-25 (precursors) and a microwave enhanced soft chemical process. Temperature was used as a variable, and only low process temperatures (100-110 C) were used. A combination of anatase nanoparticles/hydrogen tetratitanate nanotubes was synthesized using TiO2 (anatase) and a temperature of only 100 C. When TiO2 (P-25) was used with the same concentration of alkaline solution (1MNaOH), the same processing time of 12 h, and a higher temperature at 110 C, only hydrogen tetratitanate nanotubes were observed. The linkages of “Ti-O” play a very important role in the
structural features of different phases. |
|
[ Langmuir (2009) 25, (13), 7623-30 ] |
|
|
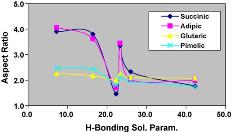
Linear models for prediction of ibuprofen crystal morphology based on hydrogen bonding propensities |
|
|
Critical to crystallization chemical product design is the choice of an appropriate solvent. Traditional methods have focused on bench scale experiments using classes of solvents (e.g. polarity) with the different classes giving rise to different crystal morphologies. However, there are instances where some solvents belonging to a particular class give completely different morphology from other solvents in the same class. There has been some modeling effort aimed at predicting crystal morphology. Amajor drawback with some of these morphology prediction models is that they tend to be limited in application. It is clear that the solvent selection, with respect to crystal morphology cannot be carried out efficiently by just experimentation or modeling alone. This paper outlines a systematic methodology which combines targeted bench scale crystallization experiments, an efficient computer-aided molecular design (CAMD) approach and a database search approach for the design and selection of solvents for crystallization of carboxylic acids. |
|
|
[ Fluid Phase Equilib. 2009, 277, (1), 73-80 ] |
|
|
…………………………………………………………………………………………………………………………………………………………………… |
|
|
Solvent design for crystallization of carboxylic acids |
|
|
|
A phase transition was achieved from TiO2 particles to titanate nanotubes by the breakage of Ti-O bonds without using expensive precursors, high temperatures, high chemical concentrations of alkaline solutions, and long synthesis times. The controlled preparation of one-dimensional nanostructures of titanates (H2Ti4O9 3 nH2O) has been conducted at a very low concentration of alkaline solution (1MNaOH), and in a very short time (12 h) using TiO2 anatase and TiO2 P-25 (precursors) and a microwave enhanced soft chemical process. Temperature was used as a variable, and only low process temperatures (100-110 C) were used. A combination of anatase nanoparticles/hydrogen tetratitanate nanotubes was synthesized using TiO2 (anatase) and a temperature of only 100 C. When TiO2 (P-25) was used with the same concentration of alkaline solution (1MNaOH), the same processing time of 12 h, and a higher temperature at 110 C, only hydrogen tetratitanate nanotubes were observed. The linkages of “Ti-O” play a very important role in the
structural features of different phases. |
|
[ Comput. Chem. Eng. 2009, 33, (5), 1014-1021 ] |
|
|
…………………………………………………………………………………………………………………………………………………………………… |
|
|
Determination of hexavalent chromium in NAVAIR trivalent chromium process (TCP) coatings and process solutions |
|
|
Two methods were used in this study to det. presence and quantities of hexavalent Cr produced by the TCP process when applied to AA2024T3. A hexavalent chrome- contg. process, Alodine 1200S, was used as the ref. One method analyzed for surface Cr6+ on coated panels and the other for its presence in treatment solns. In TCP- coated samples, no hexavalent Cr was detected even after exposure of coated samples to AS TM G 85 SO2 salt spray for up to 744 h. Similarly, none was detected after exposure to indoor or outdoor environments. Also no hexavalent Cr is produced in the TCP treatment soln. or in treated parts under prodn. conditions. This is corroborated by analyzing parts and solns. from prodn. plants treating various Al alloys >18 mo with no soln. changeover. Surface anal. of Alodine 1200S- coated panels shows 0.81 plg /cm2 of Cr6+. Amts. detected during accelerated corrosion vary depending on the corrosive environment and length of treatment time. These ranged from nondetectable after short but aggressive corrosion exposure, to detectable low levels after extended exposure. |
|
|
[ Met. Finish. (2009) 107, (2), 28-31, 33-34 ] |
|
|
…………………………………………………………………………………………………………………………………………………………………… |
|
|
Superwetting nanowire membranes for selective absorption |
|
|
|
The construction of nanoporous membranes is of great technological importance for various applications, including catalyst supports, filters for biomolecule purification, environmental remediation and seawater desalination1–3. A major challenge is the scalable fabrication of membranes with the desirable combination of good thermal stability, high selectivity and excellent recyclability. Here we present a selfassembly method for constructing thermally stable, freestanding nanowire membranes that exhibit controlled wetting behaviour ranging from superhydrophilic to superhydrophobic.
|
|
[ Nature Nanotechnology ( 2008 ), 3(6), 332-336 ] |
|
|
…………………………………………………………………………………………………………………………………………………………………… |
|
|
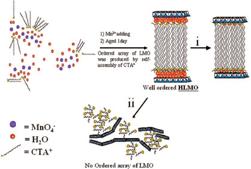
A Designed Single-Step Method for Synthesis and Structural Study of Organic-Inorganic Hybrid Materials: Well-Ordered Layered Manganese Oxide Nanocomposites |
|
|
|
Here, we designed a single-step method for rapid (1 day) preparation of well-ordered organic-inorganic hybrid LMO nanocomposites under mild conditions. This shortcut not only significantly simplifies the conventional 3-4 steps into one step but allows the possibility of large scale production of these materials due to the mild synthetic conditions, performed without high pressures
and temperatures. |
|
[ Journal of the American Chemical Society ( 2008 ), 130(44), 14390-14391 ] |
|
|
…………………………………………………………………………………………………………………………………………………………………… |
|
|
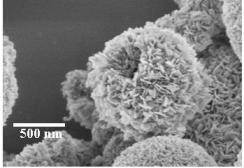
Controlled Synthesis of Self-Assembled Metal Oxide Hollow Spheres Via Tuning Redox Potentials: Versatile Nanostructured Cobalt Oxides |
|
|
|
A general method to produce nanostructured self-assembled metal oxides with hollow cores was successfully developed by controlling the net redox potentials. Cobalt and cerium oxides are demonstrated in this study. The nanostructured CoOOH hollow spheres are versatile precursors for various cobalt oxide datives (eg. Co3O4 , LiCoO2), and also possess excellent catalytic activity.
|
|
[Applied Catalysis A: General 2008, 335, 187–195] |
|
|
…………………………………………………………………………………………………………………………………………………………………… |
|
|
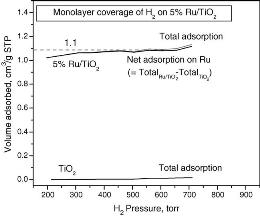
Behavior of H2 chemisorption on Ru/TiO2 surface and its application in evaluation of Ru particle sizes compared with TEM and XRD analyses |
|
|
|
Hydrogen adsorption on Ru surfaces of Ru/TiO2 catalysts has been investigated in a gas adsorption apparatus at temperatures from 35 to 150 °C, H2 partial pressures from 0.05 to 700 Torr, and adsorption equilibration time at each pressure from 3 to 60 min. Results showed that adsorption of H2 on the TiO2 support are negligible. TEM measurements suggested that there was no strong metal–support interaction (SMSI) on the synthesized Ru/TiO2 samples to inhibit H2 adsorption. A transition pressure point of 60 Torr was observed for H2 adsorption on Ru/TiO2. Most of the strong
chemisorption occurs before this transition pressure and weak chemisorption happens thereafter. H2 adsorption increases with temperatures from 35 to 75 °C indicating an activation energy present for H2 chemisorption on Ru. Above 75 °C, the adsorption slightly decreases with further increase of temperature up to 150 °C. Monolayer coverage was attained at 75 °C for 30 min equilibration time with a H2 pressure higher than 300 Torr. Monolayer H2 chemisorption was used to determine Ru metal particle size in Ru/TiO2 systems compared with XRD and TEM analyses. Selected area electron diffraction (SAD) indicated that there was no preferred crystallographic orientation of the TiO2 supported Ru. Therefore, exposed Ru atoms equally contribute from the three low-index planes [(0 0 1), (1 0 0), and (1 1 0)] with the highest atomic density instead of only the (1 0 0) plane for conventional treatments.With this assumption, the average Ru particle size calculated from H2chemisorption (4.6 nm) agrees with the TEM measurements (4.1 nm). |
|
[Applied Catalysis A: General 2008, 335, 187–195] |
|
|
…………………………………………………………………………………………………………………………………………………………………… |
|
|
Tuning of Texture and Structure of Copper-Containing Nanocomposite Oxide Materials |
|
|
|
For studies of textural tuning, structural tuning, or materials sintering, copper/aluminum and copper/zinc nanocomposite materials were prepared. Resistance to sintering of different phases was investigated. Thermal analysis methods were used to design feasible thermal treatment methods that can avoid destructive damages to gels. X-ray diffraction and nitrogen sorption measurements were used for structural and textural analysis.
Compared with the wide distributions of pore sizes and low surface areas of the products prepared via conventional coprecipitation methods, a novel urea-gelation/thermal-modification method was developed to produce CuO/Al2O3 nanocomposites with narrow distributions of pore sizes and high surface areas. In comparison with the products of conventional coprecipitation methods, this novel urea-gelation/thermalmodification method produces copper/aluminum nanocomposites with significantly higher specific copper loading, which should be valuable in apparatus that have space limitations, such as vehicle fuel cell systems. Stepwise reduction and reoxidation were studied for the structural tuning and purification of Cu-Al-O spinel phases with isotropic and gradual unit-cell contractions. The textural and structural features of some copper/ aluminum nanocomposite materials were observed by electron microscopy methods, that is, field-emission scanning electron microscopy (FESEM), high-resolution transmission electron microscopy (HRTEM), and convergent beam electron diffraction (CBED). |
|
[ Journal of Physical Chemistry C 2008, 112(5), 1446-1454 ] |
|
|
…………………………………………………………………………………………………………………………………………………………………… |
|
|
Ultrasonic Nozzle Spray in Situ Mixing and Microwave-Assisted Preparation of |
|
|
|
Nanocrystalline spinel nickel ferrite and zinc aluminate particles (6-20 nm) can be prepared by a recently developed continuous flow method that combines microwave heating and in situ ultrasonic nozzle spray mixing. The preparations were carried out at ambient pressure (1 atm), microwave power (0-600 W), and ultrasonic nozzle with resonant frequency of 48 or 120 kHz. The products were characterized by X-ray diffraction, field emission scanning electron microscopy, transmission electron microcopy, X-ray electron dispersive spectroscopy, Brunauer-Emmett-Teller spectroscopy, Raman spectroscopy, Fourier transform infrared spectroscopy, thermogravimetric analysis, and temperature-programmed desorption mass spectrometry. The results showed that the ultrasonic nozzle and microwave irradiation complement each other, with respect to the purity of the products. The specific advantages of INM method for the preparing nickel ferrites are that pure materials with high surface areas and tunable particle sizes are produced, and this process is continuous.
|
|
[J. Phys. Chem. C, 2008, early view] |
|
|
…………………………………………………………………………………………………………………………………………………………………… |
|
|
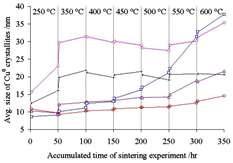
Inorganic synthesis for the stabilization of nanoparticles: application to Cu/Al2O3 nanocomposite materials |
|
|
|
A series of copper/aluminum nanocomposites with different mixing homogeneities and different texture features were prepared via various inorganic synthesis methods including coprecipitation, gelation, and stepwise thermal modification. Nitrogen sorption measurements and X-ray diffraction were used for textural and structural analysis, respectively. Solid-solid reaction analysis and differential scanning calorimetry (DSC) analysis were developed for the determination of the mixing homogeneities of copper-based nanocomposite materials. A sintering experiment at 250-600 °C for 350 h under methanol-steam reforming conditions was carried out to compare the stability of supported Cu0 nanoparticles. Although a large initial size of supported nanoparticles is not favorable, those supported nanoparticles with a small initial size cannot ensure good thermal stability. The mixing homogeneities of CuO/Al2O3 mixed metal oxide (MMO) nanocomposites significantly affected the thermal stability of their reduced Cu0 crystallites. Besides homogeneity control, creation of narrow distributions of pore sizes with small major pore diameters (e.g., around 3.5 nm) can also be used for the stabilization of supported Cu0 nanoparticles. We found
that mixing homogeneity of a nanocomposite is likely the major factor in the stabilization of nanoparticles, whereas a narrow distribution of pore sizes might confine the growth of nanoparticles, possibly via space limitations. Field-emission scanning electron microscopy (FESEM), high-resolution transmission electron microscopy (HRTEM), and convergent beam electron diffraction (CBED) were used for the observation or structure determination of the copper/aluminum nanocomposites. This paper also provides information on the deactivation of copper catalysts via thermal sintering under methanol-steam reforming conditions. |
|
[Chemistry of Materials, 2007, 19(19), 4820-4826] |
|
|
…………………………………………………………………………………………………………………………………………………………………… |
|
|
New Synthetic Route , Characterization, and Electrocatalytic Activity of Nanosized Manganite |
|
|
|
Nanosized g-MnOOH (manganite) has been synthesized by a new route via the reduction of KMnO4 with sucrose and MnSO4 in acidic medium under refluxing conditions for 4 and 6 h. Different characterization techniques of these manganite materials were used XRD, FESEM, TEM, TGA, and IR. Two new synthetic methods were developed, one involving addition of KMnO4 into a solution of both sucrose and MnSO4 while the other involved addition of KMnO 4 solution into sucrose only followed by addition of MnSO4 (s). The latter method yielded smaller particles (up to 30 nm) than the former method (up to 80 nm) and the conventionally prepared manganite (up to 50 nm). The synthesized manganite materials exhibited promising characteristics when tested as electrocatalysts in the reduction of O2.
|
|
[Chem. Mater., 2007 , 19, 1832-1839] |
|
|
…………………………………………………………………………………………………………………………………………………………………… |
|
|
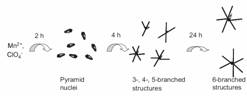
Hydrothermal Growth of Manganese Dioxide into Three-Dimensional Hierarchical Nanoarchitectures |
|
|
|
Novel three-dimensional (3D) hierarchical nanoarchitectures of e-MnO2have been synthesized by a simple chemical route without the addition of any surfactants or organic templates. The self-organized crystals consist of a major e-MnO2 dipyramidal single crystal axis and six secondary branches, which are arrays of single-crystal e-MnO2 nanobelts. The growth directions of the nanobelts are perpendicular to the central dipyramidal axis, which shows sixfold symmetry. The shape of the e-MnO2 assembly can be controlled by the reaction temperature. The morphology of e-MnO2 changes from a six-branched star-like shape to a hexagonal dipyramidal morphology when the temperature is increased from 160 to 180 °C. A possible growth mechanism is proposed. The synthesized e-MnO2 shows both semiconducting and magnetic properties. These materials exhibit ferromagnetic behavior below 25 K and paramagnetic behavior above 25 K. The e-MnO2 system may have potential applications in areas such as fabrication of nanoscale spintronic materials, catalysis, and sensors.
|
|
[Adv. Funct. Mater. 2006 , 16 , 549�555] |
|
|
…………………………………………………………………………………………………………………………………………………………………… |
|
|
Shape-Controlled Synthesis of Manganese Oxide Octahedral Molecular Sieve Three-Dimensional Nanostructures |
|
|
|
We successfully synthesized novel OMS-2 nanostructures under mild and organic template-free conditions. The shape evolution of OMS-2 can be performed via simply varying temperature. The hierarchically ordered nanoscale architectures of OMS-2 originated from primary microporous structures of cryptomelane-type manganese oxides, secondary structures of uniform nanoparticles, and tertiary architectures of microscopic arrays. These OMS-2 nanoparticles with specific structures may find potential applications in sensors, catalysis, biomarkers, microelectronics, and energy storage. |
|
[Journal of the American Chemical Society, 2005, 127(41), 14184-14185. ] |
|
|
…………………………………………………………………………………………………………………………………………………………………… |
|
|
Synthesis and Characterization of Silicon Nanowires on Mesophase Carbon Microbead Substrates by Chemical Vapor Deposition |
|
|
|
Silicon nanowires (SiNWs) have been fabricated by chemical vapor deposition at ambient pressure using SiCl4 as a silicon source and mesophase carbon microbead powder as a substrate without any templates and/or metal catalysts. The SiNWs have a crystalline core with a very thin amorphous SiOX sheath. The obtained SiNWs are homogeneous with average diameters below 50 nm and lengths up to micrometers. Temperature and time effects on the growth of SiNWs were systematically studied. Higher reaction temperatures and longer reaction times resulted in larger diameters and higher yields of SiNWs. SiNWs with a better crystallinity can be obtained at higher temperatures and longer reaction times. The obtained SiNWs were characterized by field-emission scanning electron microscopy, X-ray diffraction, Raman spectroscopy, and transmission electron microscopy.
|
|
[J. Phys. Chem. B, 2005, 109 ( 8 ), 3291 – 3297 |
|
|
…………………………………………………………………………………………………………………………………………………………………… |
|
|
Synthesis of mordenite nanocrystals
|
|
|
|
Mordenite with crystal diameter <100 nm has been prepared by the modification of different synthetic parameters such as the source of aluminum, the presence of seeds, the use of low temps., longer crystalization times, and different silica to alumina ratios. The decrease in the crystal diameter of the prepared mordenite was monitored by the application of the Scherrer equation that relates the broadness of the x-ray diffraction peaks to crystal sizes.
|
|
[Microporous and Mesoporous Materials, 2004, 67, 19-26] |
|
|
…………………………………………………………………………………………………………………………………………………………………… |
|
|
Self-Assembly of OMS Hexagonal Flakes into Mesoporous Hollow Nanospheres
|
|
|
|
Manganese oxide hollow nanospheres were prepared using a straightforward, template-free synthesis. The resulting material was mesoporous, crystalline, and of uniform diameter. The nanospheres were characterized by XRD, HR-SEM, and HR-TEM, and pore size distributions were calcined from nitrogen desorption. Unlike previous synthesis methods that use an inorganic template, this procedure requires no separation after synthesis to remove the template. The nanospheres are composed of hexagonal g-manganese oxide flakes and are approx. 400 nm in diameter g-MnO2 is composed of a ramsdellite matrix (1 x 2 tunnels) with randomly distributed microdomains of pyrolusite (1 x 1 tunnels). These materials could have applications as cathodic battery materials, oxidation.catalysts, catalyst supports, and adsorbents for pollutants.
|
|
[Journal of the American Chemical Society, 2003, 125, 4966-4967] |
|
|
…………………………………………………………………………………………………………………………………………………………………… |
|
|
Dynamic organization of inorganic nanoparticles into periodic micrometer-scale patterns
|
|
|
|
We report the study of oscillatory phenomena, in which inorg. manganese oxide nanoparticles are organized with a high degree of periodicity with multiscale ordering.
|
|
[Angewandte Chemie International Ed., 2003, 42, 2905-9] |
|
|
…………………………………………………………………………………………………………………………………………………………………… |
|
|
Magnesium Manganese Oxide Nanoribbons
|
|
|
|
A todorokite-type magnesium manganese oxide molecular sieve material with a morphology of nanoribbons has been synthesized using a combination of techniques consisting of a sol-gel process to synthesize a nanosized tetraethylammonium manganese oxide layered material, an ion-exchange method to prep. a thin-film Mg-buserite (Mg-OL-1) precursor, and a hydrothermal reaction to transform the Mg-buserite to nanoribbon-like Mg-todorokite (Mg-OMS-1) material. The transformation of the Mg-buserite thin films to Mg-todorokite nanoribbons has been studied using XRD and TEM. XRD data indicate that the structural transformation of Mg-OL-1 to Mg-OMS-1 is completed after hydrothermal heating at 150 °C for about 40 h and at 200 °C for less than 8 h. TEM data show that the nanoribbons form at an early stage of the hydrothermal treatment, followed by the appearance of lattice fringes. TEM data also indicate that the well-formed nanoribbon-like crystals have a uniform tunnel dimension along the a axis and a long-range-ordered 3 ´ 3 tunnel structure along the b axis. FESEM data show that the lengths of the nanoribbons range from a few micrometers to tens of micrometers and that the widths range from 20 to 100 nm. Elemental and average oxidation state analyses indicate a formula for the nanoribbon of Mg2.11Mn5.46O12xH2O. According to TGA and TPD-MS data, these nanoribbons are thermally stable up to 500 °C . The BET surface area is 81 m2/g, which is higher than that of bulk todorokite synthesized using conventional methods. The catalytic performance of nanoribbon-like Mg-todorokite materials on converting benzyl alcohol to benzaldehyde was improved greatly as compared to that of bulk Mg-todorokite materials. |
|
[Journal of Physical Chemistry B, 2002, 106, 9761-9768] |
|
|
…………………………………………………………………………………………………………………………………………………………………… |
|
|
Colloids, helices, and patterned films made from heme proteins and manganese oxide
|
|
|
|
New colloidal materials with enzyme-like peroxidase activity were made from octahedral layered manganese oxide and myoglobin and Hb, and were converted to macroscopic helixes and patterned films.
|
|
[Chemical Communications, 2002, 19, 2254-2255] |
|
|
…………………………………………………………………………………………………………………………………………………………………… |
|
|
Synthesis and Characterization of Nanofibrous Sodium OMS
|
|
|
|
Na Mn oxide octahedral mol. sieves with a 2 x 4 tunnel structure (Na-2 x 4) were hydrothermally synthesized from Na-birnessite materials at low temperatures and pressures. The synthetic template materials, the pH value of the medium, and the autoclaved temperature are critical in the synthesis. Na salts, such as NaCl, NaNO3, and Na2SO4, are good templates for Na-2 x 4. In strong basic solution or <160 °C, Na-birnessite does not transform to the Na-2 ´ 4 structure. TEM images show the synthesized Na-2 x 4 is made up of thin slab-like single crystals elongated along the b axis. The formula of Na-2 x 4 can be written as Na0.33Mn3+0.33Mn4+0.67O2 × xH2O, and it is monoclinic with space group C2/m. The unit cell parameters (a, b, c, and b ) for Na-2 x 4 are 14.434(5) .ANG., 2.849(7) .ANG., 23.976(6) .ANG., and 98.18 ° , respectively. These data for Na-2 ´ 4 are slightly different from the data for Rb-2 ´ 4 synthesized under high pressure and high temp., which are reported by Rziha et al. (Eur. J. Miner. 1996, 8, 1155-1161). The surface area of Na-2 x 4 is .apprx.57 m2/g. Na-2 x 4 materials are thermally stable up to 450 °C as indicated by TGA and TPD data.
|
|
[Chemistry of Materials, 2001, 13, 1585-1592] |
|
|
…………………………………………………………………………………………………………………………………………………………………… |
|
|
A review of zeolite-like porous materials
|
|
|
|
A review with 50 references on the most recent reports of zeolite-like microporous and mesoporous materials and in particular their synthesis. We focus on porous transition metal oxides of various types, also including porous pillared interlayer materials that have either pos. or neg. charges. This review covers the most recent papers published from about Jan. 1995 to the present. A discussion of new transition metal oxides, porous pillared materials, sulfide layered materials and porous phosphates is included. The section on porous transition metal oxides focuses on both microporous and mesoporous transition metal oxides and, in particular, synthetic strategies used to produce new materials. The next section concerns pillared materials, including layers that are anionic as well as cationic. Sulfide systems discussed in a further section are primarily layered phases. The final section concerns porous phosphate materials.
|
|
[Microporous and Mesoporous Materials, 2000, 37, 243-252] |
|
|
…………………………………………………………………………………………………………………………………………………………………… |
|
|
Spontaneous formation of inorganic helices
|
|
|
|
The authors show that stable helixes of porous manganese oxide materials can be formed spontaneously from uniform sols of tetra-Me ammonium (TMA) permanganate and that they are excellent semiconductors. These inorganic helixes contain micropores, can be converted into other structures, and their compn. can be varied. Ion exchange of TMA helix with K+ leads to crystalline helixes with composition K+0.93Mn4+2.1Mn3+1.9O7(OH)1.03×2.7H2O. Thermal treatment of the crystalline helixes leads to formation of an octahedral mol. sieve (OMS) tunnel structure of synthetic cryptomelane (OMS-2), and a composition of K+1.86Mn4+4.7Mn3+3.3O14.57(OH)1.43 × 0.7H2O (K-OMS-2) with an av. oxidation state for Mn of 3.58. The microstructure of K-OMS-2 helixes obtained by scanning and transmission electron microscopy indicates microporosity of the 2 ´ 2 crystal structure of OMS-2, mesoporosity in between the colloidal crystallites, and a net alignment and connectivity of the microporous network along the length of the helix. This alignment leads to conduction along the helix and, as a result of the porosity, rate processes such as ion exchange are enhanced owing to fast diffusion. The anisotropic DC-4 probe condition of 4.2 ´ 10-1 W-1cm-1 at 21 ° for the K-OMS-2 system is roughly an order of magnitude more conductive than most well-formed single crystals of semiconducting cryptomelane-like materials. |
|
video of the formation of inorganic helices |
|
|
[Nature, 2000, 405, 38] |
|
|
…………………………………………………………………………………………………………………………………………………………………… |
|
|
Films of manganese oxide nanoparticles with polycations or myoglobin from alternate-layer adsorption
|
|
|
|
Alternate adsorption of MnO2 nanoparticles with polycations poly(dimethyldiallylammonium) (PDDA) or myoglobin (Mb) onto Ag, quartz, and rough pyrolytic graphite gave stable, porous, ultrathin films. Quartz crystal microbalance (QCM) and UV-vis absorbance revealed regular film growth at each adsorption step for MnO2 and PDDA and for SiO2 nanoparticles and Mb. SEM of MnO2/PDDA films showed smooth surfaces on the 20 nm scale and cross sections consistent with individual nanoparticles. QCM during growth of films of Mb and MnO2 reflected a competition for adsorption of the protein by the film surface and dispersed MnO2 nanoparticles. Nevertheless, films of Mb and MnO2 up to 30 nm thick on rough pyrolytic graphite electrodes could be constructed. Nevertheless, films of Mb and MnO2 up to 30 nm thick on rough pyrolytic graphite electrodes could be constructed. These novel films featured reversible interconversion of the protein’s heme FeIII/FeII redox couple with 10 electroactive layers of protein, considerably more than for polyion-Mb films on smooth Au (ca. 1.3 electroactive layers), and coiled PSS/Mb films on rough graphite (7 electroactive layers). Shifts in redox potential caused by CO complexation of the heme FeII, BET specific areas, and electrochemical driven catalytic reduction of O suggest that the Mb/MnO2films are highly porous to gas molecules. These films represent the first nanofabrication of inorganic particles with functional proteins by the layer-by-layer method.
|
|
[Langmuir, 2000, 16, 8850-8857] |
|