
Mesoporous oxides attract a great deal of interest in many fields, including energy, catalysis and separation, because of their tunable structural properties such as surface area, pore volume and size, and nanocrystalline walls. Here we report thermally stable, crystalline, thermally controlled monomodal pore size mesoporous materials. Generation of such materials involves the use of inverse micelles, elimination of solvent effects, minimizing the effect of water content and controlling the condensation of inorganic frameworks by NOx decomposition. Nanosize particles are formed in inverse micelles and are randomly packed to a mesoporous structure. The mesopores are created by interconnected intraparticle voids and can be tuned from 1.2 to 25 nm by controlling the nanoparticle size. Such phenomena allow the preparation of multiple phases of the same metal oxide and syntheses of materials having compositions throughout much of the periodic table, with different structures and thermal stabilities as high as 800 °C.
[Nat. Commun. 4, 2952 (2013)]

An inverse-micelle sol–gel method was used to prepare Ti and Fe-doped mesoporous silica catalysts, and they were utilized for selective oxidation of styrene to benzaldehyde. The amorphous peak of silica was confirmed by XRD and there were no peaks related to Ti or Fe oxides. Results indicate that the metals were homogeneously distributed in the silica matrix, leading to higher surface area and pore volume. Introduction of Ti species into mesoporous silica improved the total catalyst acidity and catalytic data revealed that the oxidation activity of Ti-doped mesoporous silica (Ti-MS) catalyst achieved 93% styrene conversion and 91% benzaldehyde selectivity under optimized conditions. Formic acid, phenylacetaldehyde, acetophenone and epoxy-styrene are all minor products of this reaction. The acid strength and the use of appropriate solvents and oxidants are crucial to achieving high styrene conversion and benzaldehyde yield. The catalysts were reusable up to 4 cycles without loss of activity.
[Journal of Colloid and Interface Science, 2025, 679(B), 1063-1078]
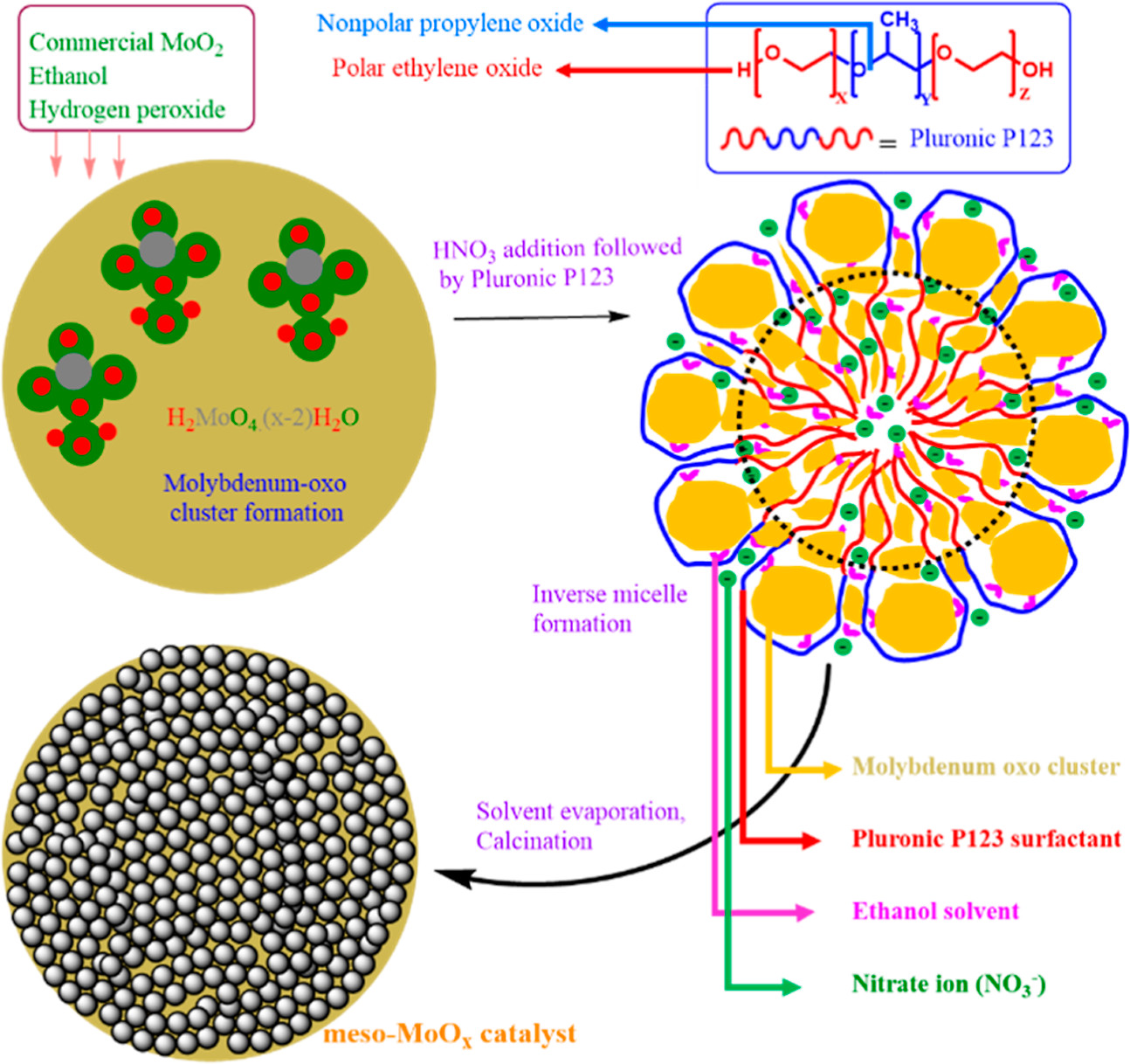
Herein, a straightforward synthesis method for highly mesoporous molybdenum oxide has been demonstrated via use of inverse micelles and molybdenum-oxo cluster formation. The synthesized catalyst is stable, crystalline, and MoO3 phase pure, as confirmed through thermogravimetric analysis, X-ray diffraction, and X-ray photoelectron spectroscopy. Further results from electron paramagnetic resonance, Raman spectroscopy, and UV–vis spectroscopy confirm the MoO3 phase purity. Chemisorption studies reveal that the synthesized material is 65 times more active than its commercial parts. The quantitative value of ammonia chemisorption for the synthesized catalyst is 1270 μmol/g, whereas the commercial catalyst only gives 22 μmol/g. These materials were tested for electrophilic substitution reactions since they are excellent solid acid. Electrophilic substitution of benzyl alcohol with toluene gives a >99% conversion with ∼80% of selectivity toward the methyl diphenylmethane product. The turnover number and turnover frequency values were calculated to be as high as 115 and 38, respectively. A substrate scope study shows that the reaction has preference toward electron-donating groups, whereas electron-withdrawing groups block the reaction. Based on the obtained results, a mechanism has been proposed.
[ACS Appl. Mater. Interfaces, 2022, 14(45), 51041-51052]
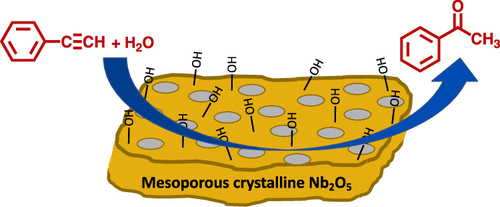
A mesoporous crystalline niobium oxide with tunable pore sizes was synthesized via the sol–gel-based inverse micelle method. The material shows a surface area of 127 m2/g, which is the highest surface area reported so far for crystalline niobium oxide synthesized by soft template methods. The material also has a monomodal pore size distribution with an average pore diameter of 5.6 nm. A comprehensive characterization of niobium oxide was performed using powder X-ray diffraction, Brunauer–Emmett–Teller, thermogravimetric analysis, scanning electron microscopy, transmission electron microscopy, UV–vis, and X-ray photoelectron spectroscopy. The material acts as an environmentally friendly, solid acid catalyst toward hydration of alkynes under with excellent catalytic activity (99% conversion, 99% selectivity, and 4.39 h–1 TOF). Brønsted acid sites present in the catalyst were found to be responsible for the high catalytic activity. The catalyst was reusable up to five cycles without a significant loss of the activity.
[ACS Appl. Mater. Interfaces, 2020, 12(42), 47389-47396]
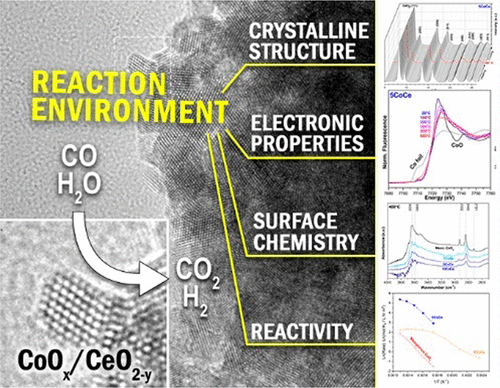
Mesoporous Co/CeO2 catalysts were found to exhibit significant activity for the high-temperature water-gas shift (WGS) reaction with cobalt loadings as low as 1 wt %. The catalysts feature a uniform dispersion of cobalt within the CeO2 fluorite type lattice with no evidence of discrete cobalt phase segregation. In situ XANES and ambient pressure XPS experiments were used to elucidate the active state of the catalysts as partially reduced cerium oxide doped with oxidized cobalt atoms. In situ XRD and DRIFTS experiments suggest facile cerium reduction and oxygen vacancy formation, particularly with lower cobalt loadings. In situ DRIFTS analysis also revealed the presence of surface carbonate and bidentate formate species under reaction conditions, which may be associated with additional mechanistic pathways for the WGS reaction. Deactivation behavior was observed with higher cobalt loadings. XANES data suggest the formation of small metallic cobalt clusters at temperatures above 400°C may be responsible. Notably, this deactivation was not observed for the 1% cobalt loaded catalyst, which exhibited the highest activity per unit of cobalt.
[J. Phys. Chem. C, 2018, 122(16), 8998-9008]

Catalytic combustion of methane at low temperature under lean conditions was investigated over mesoporous amorphous manganese oxide (Meso-Mn-A), Mn2O3 (Meso-Mn2O3), MnO2 (epsilon phase) (Meso-ϵ-MnO2), and octahedral molecular sieves MnO2 (Meso-OMS-2) synthesized using an inverse surfactant micelle method. The prepared materials are monodispersed nanoparticle aggregates, and the mesopores are formed by connected interparticle voids. All the mesoporous manganese oxides proved to be significantly active compared to nonporous, similar phase materials. However, among the tested materials Meso-Mn-A showed the lowest light-off temperature of 229 °C, but Meso-OMS-2 showed the highest conversion (90%) at the lowest temperature of 373 °C. Despite the low light-off temperatures of mesoporous materials, even nonporous K-OMS-2 (cryptomelane) showed 90% conversion at 403 °C illustrating not only the effect of mesopore size but also the oxidation state of manganese and the structure of the catalyst having effects on the activity of manganese oxides. X-ray photoelectron spectroscopy (XPS), H2-temperature-programmed reduction (H2-TPR), and N2 sorption analysis indicated that the oxidation states of catalysts, surface oxygen vacancies, and large surface areas promoted the lattice oxygen mobility of the catalysts. Thus, activities of the catalysts were correlated to the oxidation states, the lattice oxygen mobility, and the reducibility of the catalysts. The apparent activation energy of methane oxidation calculated based on a pseudo-first-order kinetics ranged from 70.5 to 107.2 kJ mol–1 for the manganese oxides, and the values are comparable with catalysts containing precious metals.
[The Journal of Physical Chemistry C, 2015, 119(3), 1473-1482]