Atomically dispersed catalysts have drawn great interest lately, as they showcase a high density of active sites, selectivity, and high turnover frequencies in oxidation chemistry due to labile oxygen activation. In contrast, the applications of these catalysts have lagged in reduction reactions due to the ambiguity caused by the sintering and restructuring of active sites. To bridge this gap, the evolution of Pt4 + isomorphically substituted into an octahedral molecular sieve structure (OMS-2) under reductive conditions was correlatively characterized using multiple in-situ analytical techniques such as ambient pressure X-ray photoelectron spectroscopy, environmental transmission electron microscopy, and solid-state nuclear magnetic resonance. The surface dynamics of the Pt single atoms were revealed during the Reverse Water Gas Shift (RWGS) reaction, where the active sites were identified as two-coordinated platinum single atoms. Under reaction, we show nonbinding atoms adjacent to the single atoms restructured the motif of the single atoms to Pt2+ via ion mobility of potassium, increasing the activation energy by 25.6 kJ/mol. This work also highlights the potential for increased stability of the single atom sites via isomorphic substitution of the metal oxide support, since the Pt-OMS-2 catalyst retained activity for about 33 h before deactivation, after which nanoparticles were observed in TEM images. This work offers a new perspective in single atom synthesis using the metal oxide as the host for the single atom site, instead of adatoms on the surface
[Appl. Cata. B, 2026, 383, 126016]
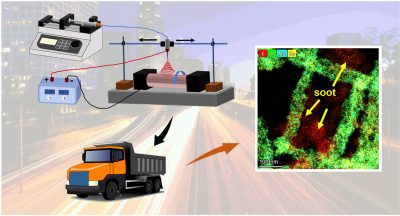
Increasing the contact efficiency and improving the intrinsic activity are two effective strategies to obtain efficient catalysts for soot combustion. Herein, the electrospinning method is used to synthesize fiber-like Ce–Mn oxide with a strong synergistic effect. The slow combustion of PVP in precursors and highly soluble manganese acetate in spinning solution facilitates the formation of fibrous Ce–Mn oxides. The fluid simulation clearly indicates that the slender and uniform fibers provide more interwoven macropores to capture soot particles than the cubes and spheres do. Accordingly, electrospun Ce–Mn oxide exhibits better catalytic activity than reference catalysts, including Ce–Mn oxides by co-precipitation and sol-gel methods. The characterizations suggest that Mn3+ substitution into fluorite-type CeO2 enhances the reducibility through the acceleration of Mn–Ce electron transfer, improves the lattice oxygen mobility by weakening the Ce–O bonds, and induces oxygen vacancies for the activation of O2. The theoretical calculation reveals that the release of lattice oxygen becomes easy because of a low formation energy of oxygen vacancy, while the high reduction potential is beneficial for the activation of O2 on Ce3+-Ov (oxygen vacancies). Due to above Ce–Mn synergy, the CeMnOx-ES shows more active oxygen species and higher oxygen storage capacity than CeO2-ES and MnOx-ES. The theoretical calculation and experimental results suggest that the adsorbed O2 is more active than lattice oxygen and the catalytic oxidation mainly follows the Langmuir-Hinshelwood mechanism. This study indicates that electrospinning is a novel method to obtain efficient Ce–Mn oxide.
[Chemosphere, 2023, 334, 138995]
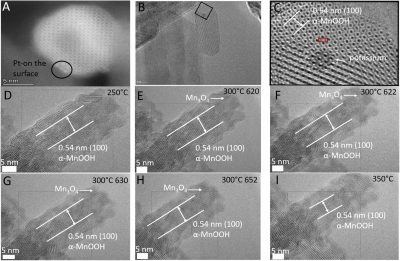
The properties of manganese oxide nanomaterials are dictated by the structure and morphology of the particular phases they can adopt. In this work, the effects of post-synthesis heat treatments on amorphous monodisperse manganese oxide nanoparticles at temperatures of up to 1300 °C under laboratory air and argon atmospheres have been investigated using X-ray diffraction, thermal analysis, and electron microscopy techniques. During heat treatments in air, the nanoparticles undergo three transformations, resulting in: crystallization to cubic Mn2O3 at ~ 500 °C, followed by the transformation to tetragonal Mn3O4 at ~ 1010 °C, and to cubic Mn3O4 at ~ 1190 °C. The first two transformations are irreversible, are associated with oxygen loss, and involve reductions of the Mn ions. The latter transformation is polymorphic and spontaneously reversible, and so, tetragonal Mn3O4 is observed at ambient temperature in samples heat-treated at above 1000 °C. The samples heat-treated in argon firstly crystallize to a mixture of monoclinic Mn2O3 and tetragonal Mn3O4 at ~ 475 °C, followed by complete transformation to tetragonal Mn3O4 at ~ 820 °C, and then to mostly cubic MnO at ~ 1145 °C; here again, the residual tetragonal Mn3O4 undergoes a reversible polymorphic transformation at ~ 1180 °C, whereas the other transformations are irreversible. In both atmospheres, the amorphous material exhibits short-range cryptomelane-type order with a mixture of Mn3+ and Mn4+ prior to crystallization. These data indicate that most of the stable manganese oxide phases can be obtained from initially amorphous nanoparticles by heat treatment under appropriate conditions.
[Journal of Mater. Sci., 2020, 55, 7247-7258]
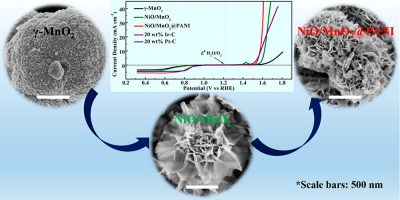
We report on the new facile synthesis of mesoporous NiO/MnO2 in one step by modifying inverse micelle templated UCT (University of Connecticut) methods. The catalyst shows excellent electrocatalytic activity and stability for both the oxygen evolution reaction (OER) and the oxygen reduction reaction (ORR) in alkaline media after further coating with polyaniline (PANI). For electrochemical performance, the optimized catalyst exhibits a potential gap, ΔE, of 0.75 V to achieve a current of 10 mA cm–2 for the OER and −3 mA cm–2 for the ORR in 0.1 M KOH solution. Extensive characterization methods were applied to investigate the structure–property of the catalyst for correlations with activity (e.g., XRD, BET, SEM, HRTEM, FIB-TEM, XPS, TGA, and Raman). The high electrocatalytic activity of the catalyst closely relates to the good electrical conductivity of PANI, accessible mesoporous structure, high surface area, as well as the synergistic effect of the specific core–shell structure. This work opens a new avenue for the rational design of core–shell structure catalysts for energy conversion and storage applications.
[ACS Appl. Mater. Interfaces, 2017, 9(49), 42676-42687]
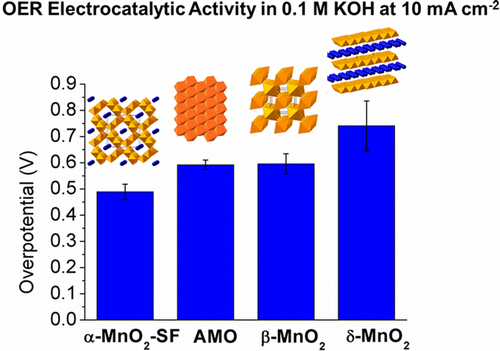
Manganese oxides of various structures (α-, β-, and δ-MnO2 and amorphous) were synthesized by facile methods. The electrocatalytic properties of these materials were systematically investigated for catalyzing both oxygen evolution reaction (OER) and oxygen reduction reaction (ORR) in alkaline media. Extensive characterization was correlated with the activity study by investigating the crystal structures (XRD, HRTEM), morphologies (SEM), porosities (BET), surfaces (XPS, O2-TPD/MS), and electrochemical properties (Tafel analysis, Koutechy-Levich plots, and constant-current electrolysis). These combined results show that the electrocatalytic activities are strongly dependent on the crystallographic structures, and follow an order of α-MnO2 > AMO > β-MnO2 > δ-MnO2. Both OER studies and ORR studies reveal similar structure-determined activity trends in alkaline media. In the OER studies, α-MnO2 displays an overpotential of 490 mV compared to 380 mV shown by an Ir/C catalyst in reaching 10 mA cm(-2). Meanwhile, α-MnO2 also exhibits stability for 3 h when supplying a constant current density of 5 mA cm(-2). This was further improved by adding Ni(2+) dopants (ca. 8 h). The superior OER activity was attributed to several factors, including abundant di-μ-oxo bridges existing in α-MnO2 as the protonation sites, analogous to the OEC in PS-II of the natural water oxidation system; the mixed valencies (AOS = 3.7); and the lowest charge transfer resistances (91.8 Ω, η = 430 mV) as revealed from in situ electrochemical impedance spectroscopy (EIS). In the ORR studies, when reaching 3 mA cm(-2), α-MnO2 shows 760 mV close to 860 mV for the best ORR catalyst (20% Pt/C). The outstanding ORR activity was due to the strongest O2 adsorption capability of α-MnO2 suggested by temperature-programmed desorption. As a result, this discovery of the structure-related electrocatalytic activities could provide guidance in the further development of easily prepared, scalable, and low-cost catalysts based on metal oxides and their derivatives.
[J. Am. Chem. Soc., 2014, 136(32), 11452-11464]
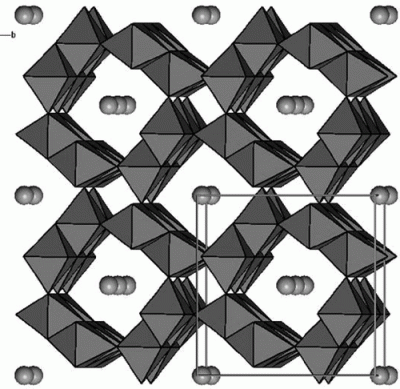
Tungsten was successfully doped at 1 and 2 mol % tungsten into the K-OMS-2 framework. Sodium tungstate and tungsten pentabromide were used in a reflux synthesis preparation. The data collected from the characterization methods collectively affirm the substitution of tungsten into the K-OMS-2 framework. Conductivity measurements showed an increase in the resistivity. Conversion of benzyl alcohol to benzaldehyde had a conversion of 25 and 15% for the sodium tungstate and tungsten pentabromide, respectively, while retaining 100% selectivity. Properties such as the resistivity, thermal stability, and crystallinity of the material were altered depending on the amount and type of starting reactants used.
[Chem. of Mater., 2008, 20(20), 6382-6388]
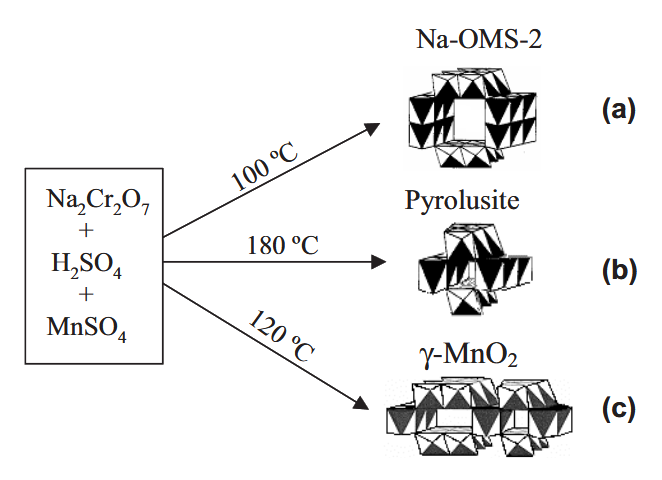
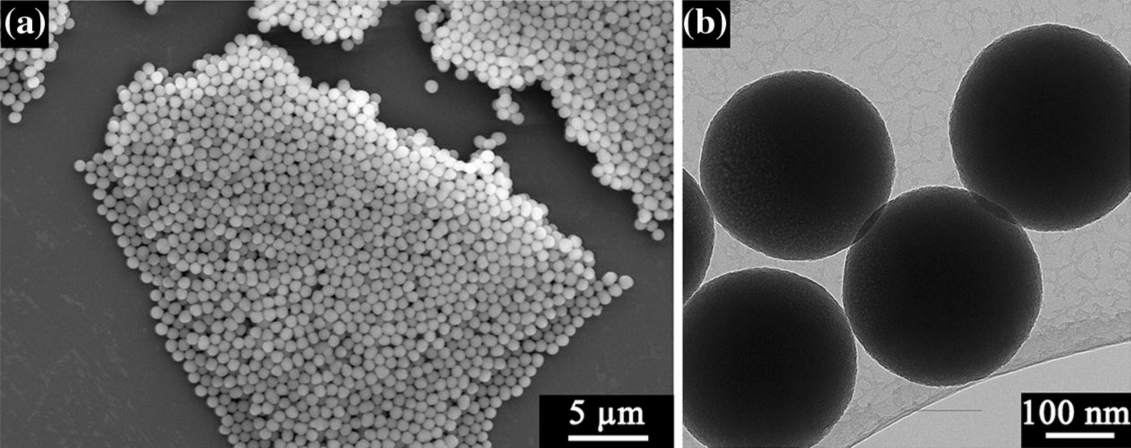
Highly uniform single-crystal Na-OMS-2 (OMS: octahedral molecular sieve), pyrolusite, and γ-MnO2 nanostructures with an interesting 3D urchinlike morphology have been successfully prepared using a hydrothermal method based on a mild and direct reaction between sodium dichromate and manganese sulfate. The crystal phases, shapes, and tunnel sizes of the manganese dioxide nanostructures can be tailored. Reaction temperature, concentrations of the reactants, and acidity of the solution play important roles in controlling the synthesis of these manganese dioxides. Field-emission scanning electron microscopy and transmission electron microscopy (TEM) studies show that the nanomaterials obtained are constructed of self-assembled nanorods. X-ray diffraction and TEM results indicate that the constituent manganese dioxide particles are single-crystalline materials. Energy dispersive X-ray analysis and magnetic studies imply that chromium cations may be incorporated into the framework and/or tunnels of the manganese dioxides. A mechanism for the growth of manganese dioxides with urchinlike architectures is proposed.
[Adv. Func. Mater., 2006, 16(9), 1247-1253]
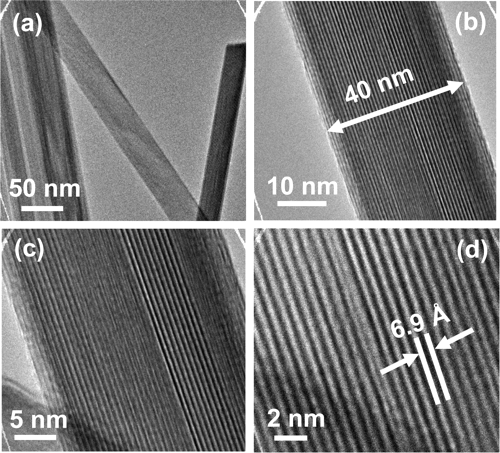
Romanechite is a natural manganese oxide with a 2 × 3 tunnel structure containing a majority of Ba2+ and trace amounts of Na+, K+, and Sr2+ as tunnel cations. Many attempts have been made to synthesize the 2 × 3 tunnel structured manganese oxide in laboratories using Ba2+ as the template. However, no successful work has been reported due to (1) the presence of intergrown hollandite impurities (barium-2 × 2 tunnel structure manganese oxide) in the synthesized products and (2) the absence of romanechite characteristic X-ray diffraction (XRD) peaks in the products, such as the (001) and (200) diffraction peaks which correspond to d ≈ 9.7 and 7.0 Å, respectively. Hydrated Na+ ions have been utilized as structure directors to successfully synthesize the Na-2 × 3 tunnel structure manganese oxide (OMS-6) from hydrothermal treatment of Na-birnessite. XRD gave a pattern in very good agreement with the pattern of romanechite (JCPDS file 14-627) without impurity phases. High-resolution microscopy measurements showed a nanofibrous morphology of the materials with an average fiber diameter of 40 nm. Under N2 environments, the 2 × 3 tunnel structure is stable below 550 °C and transforms into hausmannite (Mn3O4) at temperatures of 550°C or higher; however, under O2 environments, the 2 × 3 tunnel structure is preserved at 550°C as measured by in situ XRD. The density functional theory (DFT) method indicated that the 2 × 3 tunnel structure has a major micropore size of 7.5 Å. NH3 and CO2 chemisorption results indicated that the amount of strong acidic and basic sites on the Na-2 × 3 material is about 0.22 and 0.002 mmol/g sample, respectively. Catalytic oxidation of indene by the Na-2 × 3 manganese oxide showed a 96% conversion for indene and a 73% selectivity toward phthalic anhydride for a 40 h reaction at 80°C.
[Chemistry of Materials, 2004, 16(25), 5327-5335]