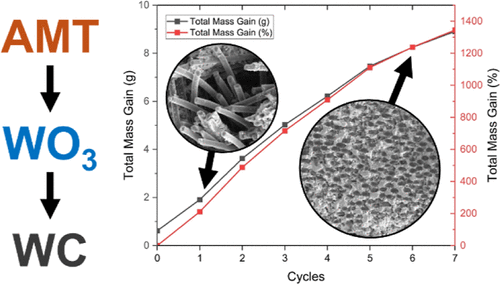
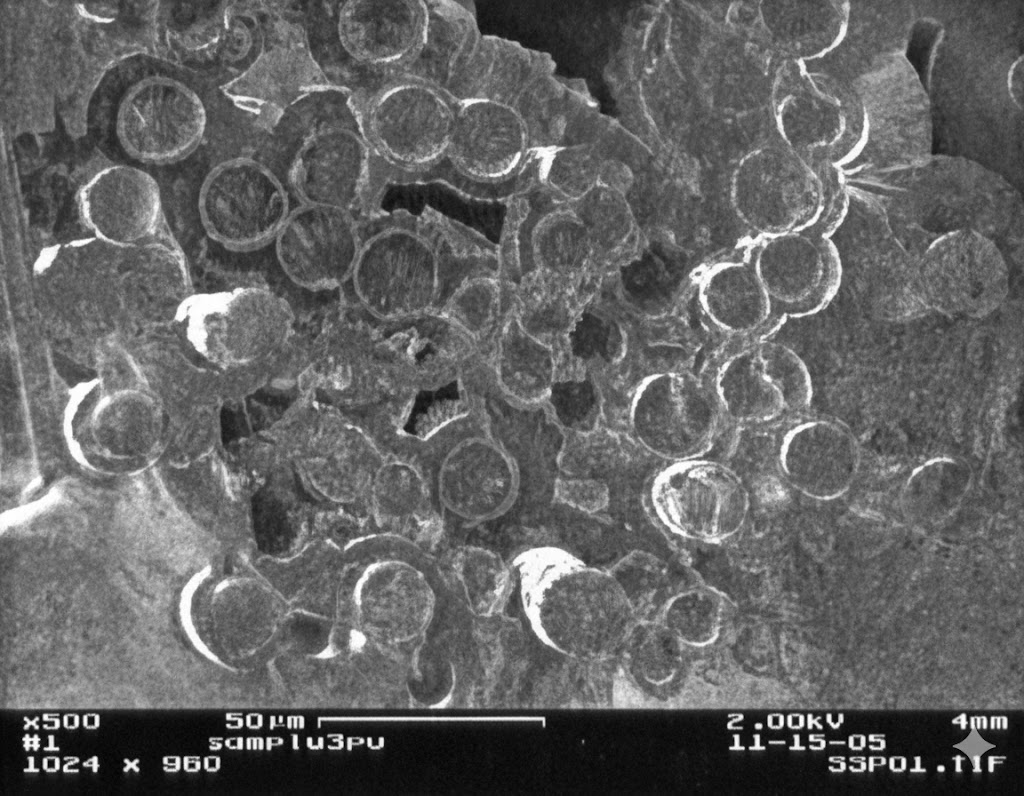
A simple, cost-effective, and easily repeatable method for the densification of tungsten carbide (WC) into nonwoven 3D carbon fiber structures was carried out. Tungsten carbide has a high melting point, high Mohs hardness, and good thermal conductivity, which makes it useful for aerospace applications. Tungsten carbide chemical vapor deposition comes at a high cost, and this method is a cost-effective primary infiltration technique to be utilized before vapor deposition. This method yields highly crystalline microparticles of tungsten carbide spread throughout the 3D structure with densification rates reaching 85% via this method alone. Both large scale (∼78.5 cm3) and small scale (1 cm3) samples were analyzed by scanning electron microscopy, X-ray diffraction, transmission electron spectroscopy, energy dispersive X-ray spectroscopy, X-ray fluorescence, and X-ray photoelectron spectroscopy post-densification. Scanning electron micrographs and X-ray diffractograms were collected for every other densification cycle.
[ACS Appl. Eng. Mater., 2025, 3(10), 3455-3463]
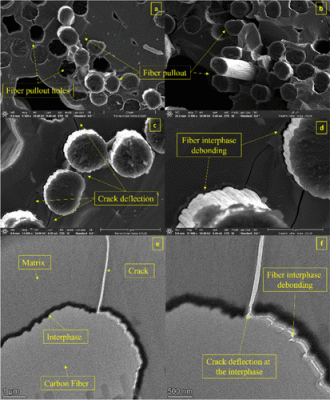
Ceramic matrix composites (CMCs) have played a significant role in increasing the efficiency of gas turbine engines. CMCs combine the high temperature resistance of ceramics with the high mechanical strength of ceramic fibers into a single unit. Interphase layers are a crucial component in CMCs, as they prevent ceramic fibers from oxidation and introduce strengthening mechanisms into the composite. Hexagonal boron nitride and pyrolytic carbon are the most commonly used interphase layers in the aerospace industry. Other than that, very few materials have been evaluated as interphase layers. In this study, we explore the possibilities of using titanium nitride as an interphase layer in single-tow CMCs (mini composite) representative of a unidirectional composite at a smaller scale. T-300 carbon fibers were coated with TiN by atmospheric pressure chemical vapor infiltration using TiCl4, N2, and H2. The deposition temperature, precursor flow rate ratio, total precursor flow rate, and deposition time were optimized to obtain high-quality coatings. The best coating was produced at 800 °C, 4:1 H2 [TiCl4]/N2 ratio, 125 standard cubic centimeters per minute (N2 + H2 [TiCl4]) total flow precursor flow rate, and 2 h of deposition time. At these conditions, the coatings displayed good fiber coverage, good fiber adhesion, minimum fiber linkage, and minimum surface roughness. There was minimum fiber degradation after TiN coating, with a retention of 95% of the initial Young’s modulus and 26% of the ultimate tensile strength of the carbon fiber. Adding the TiN interphase coating to the Cf/SiC CMC increased the ultimate tensile strength of the composite by 1122% and Young’s modulus by 150%.
[ACS Appl. Mater. Interfaces, 2024, 16(42), 57702-57714]
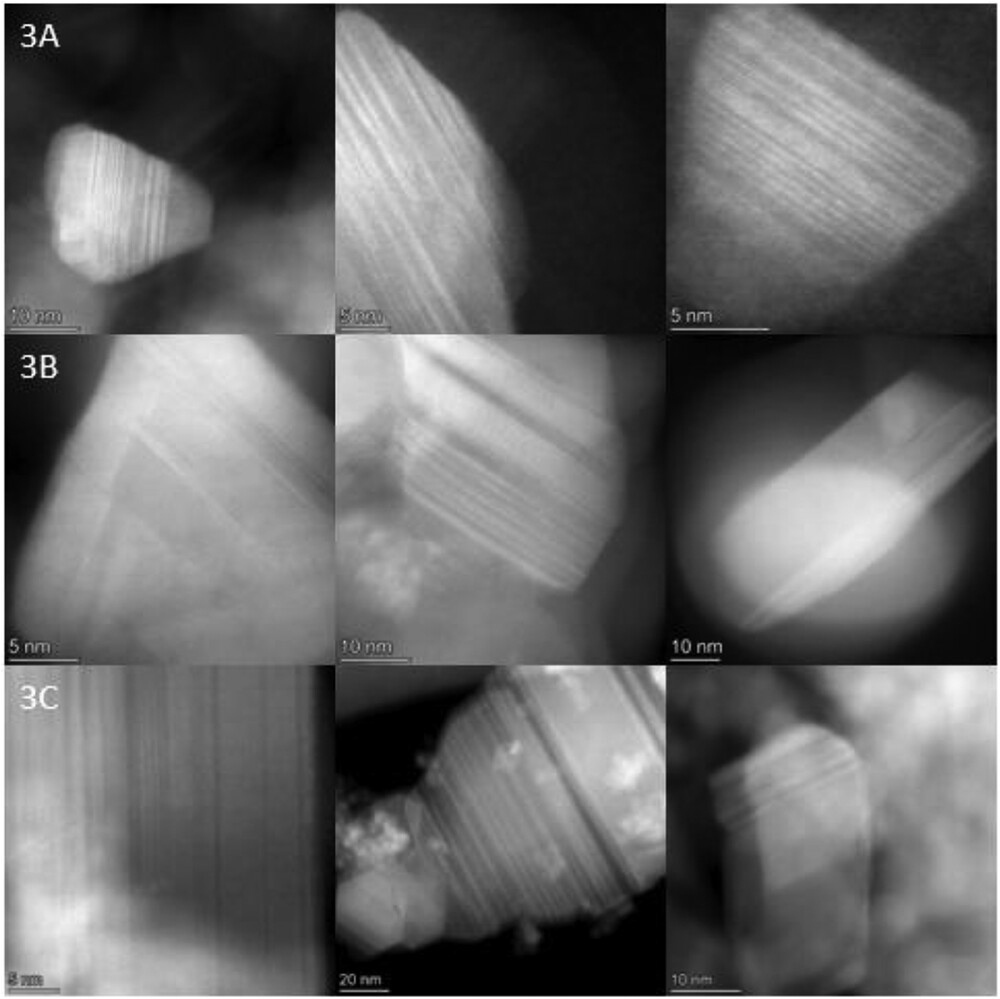
Polysilazane (PSZ) polymers offer the ability to form silicon carbide (SiC) or silicon nitride (Si3N4) via thermal pyrolysis. The majority of studies surrounding this polymer have focused on the decomposition of PSZ to amorphous glass. Investigations into the structural defects and bonding environments of the final ceramic systems are not as frequently discussed. Examining structural defects/abnormalities can help better use PSZ-derived ceramics in electronic and high-temperature applications, where phase identity and microstructure are important.
In this work, three PSZ-derived polymers were synthesized with different C/N ratios (1.33, 1.0, and 1.2) via the ammonolysis condensation reaction with chlorinated silanes. The samples were named PSZ-SiCF1-3. Samples were then pyrolyzed under Ar in a tube furnace at 1600°C for 5 h. Characterization included X-ray diffraction (XRD), Raman spectroscopy, X-ray photoelectron spectroscopy, scanning transmission electron microscopy, and electron energy loss spectroscopy.
XRD measurements uncovered arbitrary stacking fault (SF) measurements of 3C-SiC to be 4.12, 1.08, and 2.12 (unitless) for samples PSZ-SiCF1-3. Results were supported through Raman spectroscopy and TEM. High carbon content, not higher residual nitrogen content, was more impactful for SF formation. This highlights the importance of SFs in the microstructural evolution of non-oxide ceramics.
[Journal of the American Ceramic Society, 2024, 107(6), 4296-4306]
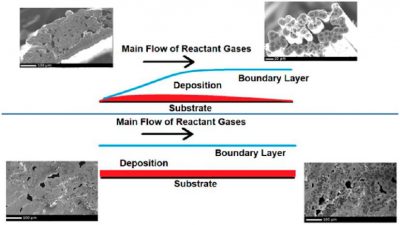
The chemical vapor infiltration technique is one of the most popular for the fabrication of the matrix portion of a ceramic matrix composite. This work focuses on tailoring an atmospheric pressure deposition of silicon carbide onto carbon fiber tows using the methyltrichlorosilane (CH3SiCl3) and H2 deposition system at atmospheric pressure to create minicomposites faster than low pressure systems. Adjustment of the flow rate of H2 bubbled through CH3SiCl3 will improve the uniformity of the deposition as well as infiltrate the substrate more completely as the flow rate is decreased. Low pressure depositions conducted at 50 Torr deposit SiC at a rate of approximately 200 nm*h−1, while the atmospheric pressure system presented has a deposition rate ranging from 750 nm*h−1 to 3.88 μm*h−1. The minicomposites fabricated in this study had approximate total porosities of 3 and 6% for 10 and 25 SCCM infiltrations, respectively.
[ACS Applied Materials and Interfaces, 2018, 10, 4986-4992]
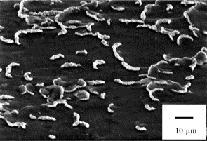
The design of a coating interface for high temperature Ceramic Matrix Composites (CMCs) is vital to achieving the high level of operating requirements for CMCs in aerospace applications. Increasing toughness and oxidative resistance, especially for SiC based fiber reinforced composites, require a coating system with strict control over thickness and processing conditions to prevent damage during deposition and promote creep resistance. A boron nitride coating was deposited on NicalonTM fabric using a Low Pressure Chemical Vapor Deposition (LP-CVD) reactor. Following previous investigations, an environmental protective layer consisting of Si3N4 was also deposited. SiC/SiC single tow unidirectional mini-composites manufactured by Chemical Vapor Infiltration (CVI), with and without the coating system, were processed. Correlations between thickness and tensile strengths of both the fiber and mini-composites are presented. Variations in BN and Si3N4 coating thicknesses represent a strong influence on overall tensile strengths of the processed fiber and mini-composites. Field-Emission Scanning Electron Microscope (FE-SEM) and Scanning Auger Spectroscopy were used in characterizing the interfacial composition and morphology. Thermo-Gravimetric Analysis was used to determine if a thin layer of Si3N4 could decrease the moisture problems that turbostratic BN exhibits. The overall coating process on Nicalon fiber did not significantly decrease the preprocessed fiber tensile strengths while successfully promoting high temperature usability in typical environments met by CMC components in aerospace applications.
[Journal of Materials Science, 2013, 48(18), 6194-6202]
Alumina thin films were coated onto nickel substrates via MOCVD. The alumina was produced by pyrolysis of aluminum acetylacetonate precursor. This reaction was done at relatively low temps., from 435-550 °C to deposit a thin film of alumina. The coating provided protection against isothermal oxidn. at elevated temps. Resistance to oxidn. was obtained for all of the coated substrates. Surface morphol., however, changed when heated for prolonged time. Samples coated at 500 and 550 °C were effective < 900 °C whereas samples coated at 435 °C were good < 800 °C. The metal oxide compns. and phases were studied by x-ray diffraction and EDAX. The surface properties of the deposited thin films were investigated with SEM.
[Surface and Coatings Technology, 2003, 173, 74-80]