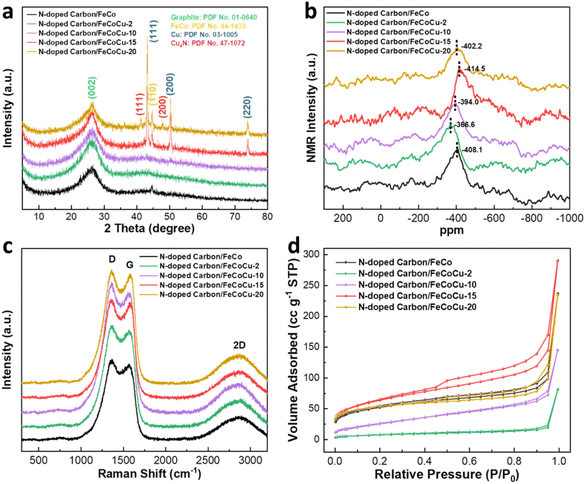
Zinc–air batteries are potential devices for energy conversion and storage, offering high energy and power density. Efficient, durable, and cost-effective electrocatalysts that accelerate sluggish oxygen reduction kinetics are crucial for achieving high performance. Here we have developed a high-performance oxygen reduction catalyst based on N-doped carbon and FeCoCu particles encapsulated in graphitic carbon nanotube composites (N-doped carbon/FeCoCu). Through the systematic experimental and simulation studies, we propose a synergetic coupling among FeCoCu nanoparticles and N-doped carbon nanotubes. The electron transfer from FeCoCu nanoparticles to carbon active sites through metal–N–C moieties affects the crystal structure, local environment, and electronic properties of the catalyst, enhancing its conductivity, electrocatalytic performance, and reaction kinetics, while also providing exceptional durability in alkaline electrolyte. Consequently, as an alternative to the precious Pt catalyst, N-doped carbon/FeCoCu catalyst used in the air cathode of zinc–air batteries exhibits remarkable specific capacities (810 mA h g−1) with large energy densities (918 W h kg−1), and a peak power density of 154.7 mW cm−2. Additionally, impressive reversibility and stability are demonstrated throughout extensive charge/discharge cycles over 900 h, holding great potential for practical applications in next-generation sustainable and green rechargeable batteries.
[J. Mater. Chem. A, 2025, 13, 14216-14228]

Titanium(IV) oxide (TiO2) is a promising alternative to graphite anodes used in Li-ion batteries (LIBs) due to its low toxicity and small volume change during cycling. SnO2 has a higher specific capacity than TiO2 but suffers from large volume changes during charging-discharging. Accordingly, doping of TiO2 with Sn can provide higher Li-ion storage capacity, while maintaining the advantages of TiO2. Here, a mesoporous Sn-doped TiO2 with high surface area (up to 259 m2/g) is synthesized using an inverse micelle sol-gel method followed by varying the calcination temperature. Crystallographic studies showed successful Sn doping. The electrochemical performance of the synthesized materials was evaluated by constructing a lithium-ion half-cell battery and all the batteries were cycled at both constant and variable charge rates. The 8 % Sn doped TiO2 calcined at 350℃ had the highest 340 mAh/g specific capacity which is twice that of the same amount of Sn-doped sample calcined at 250℃. There is a correlation between increased Li-ion storage capacity of the calcined mesoporous samples and the porosity and oxidation state of the constituent ions. The intent of this study is to show the importance of optimizing calcination temperature that may result in improved electrochemical performance of Li-ion batteries with similar anode materials, not necessarily to outperform the existing Sn-doped TiO2 samples.
[Future Batteries, 2025, 5, 100038]
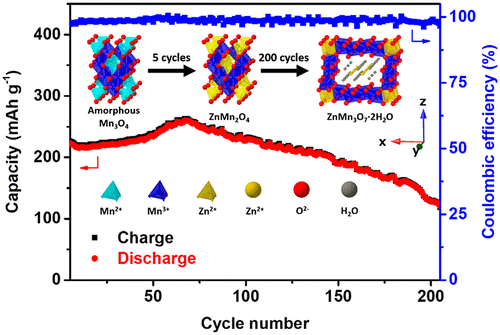
Crystalline manganese oxides have attracted the most attention in aqueous zinc-ion batteries due to their diverse nanostructures and low cost. However, extensive studies on amorphous manganese oxides are lacking. Herein, we report a mesoporous amorphous manganese oxide (UCT-1-250) as a cathode material with high capacity (222 mAh g–1), good cyclability (57% capacity retention after 200 cycles), and an acceptable discharge plateau (between 1.2 and 1.4 V). An approach to mechanistic studies was performed by comparison of UCT-1-250 and other crystalline manganese oxides through electrochemical, elemental, and structural analyses. An in situ conversion to ZnMn2O4 spinel phase after initial cycling contributes to the high performance. The irreversible capacity fading is due to the formation of the woodruffite phase.
[ACS Appl. Energy Mater., 2020, 3(2), 1627-1633]