
[Synthetic Metals, 2025, 312, 117866]
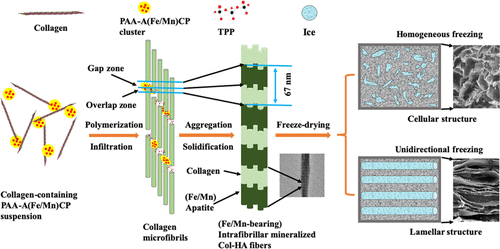
As one of the major challenges in the field of tissue engineering, large skeletal defects have attracted wide attention from researchers. Collagen (Col) and hydroxyapatite (HA), the most abundant protein and the main component in natural bone, respectively, are usually used as a biomimetic composite material in tissue engineering due to their excellent biocompatibility and biodegradability. In this study, novel intrafibrillar mineralized Col–HA-based scaffolds, constructed in either cellular or lamellar microstructures, were established through a biomimetic method to enhance the new bone-regenerating capability of tissue engineering scaffolds. Moreover, iron (Fe) and manganese (Mn), two of the essential trace elements in the body, were successfully incorporated into the lamellar scaffold to further improve the osteoinductivity of these biomaterials. It was found that the lamellar scaffolds demonstrated better osteogenic abilities compared to both in-house and commercial Col–HA-based cellular scaffolds in vitro and in vivo. Meanwhile, Fe/Mn incorporation further amplified the osteogenic promotion of the lamellar scaffolds. More importantly, a synergistic effect was observed in the Fe and Mn dual-element-incorporated lamellar scaffolds for both in vitro osteogenic differentiation of bone marrow mesenchymal stem cells (BMSCs) and in vivo bone regeneration loaded with fresh bone marrow cells. This study provides a simple but practical strategy for the creation of functional scaffolds for bone regeneration.
[ACS Appl. Mater. Interfaces, 2020, 12(16), 18235-18249]

The growth of nano-sized crystalline materials with orientational and morphological control is crucial for considerable applications, however, even when this can be achieved, the preparation techniques required are usually complex and specific to a single system. We demonstrate here an inexpensive and scaleable hydrothermal route to produce heteroepitaxial rutile TiO2 nanoscale shell-fiber superstructures (NSFS) on cryptomelane MnO2 (OMS-2) substrates. The application of this procedure to thin film cryptomelane MnO2 substrates resulted in uniform TiO2 nanofiber arrays over large areas indicating the potential of this approach for application in sensors and energy-harvesting devices. The cryptomelane MnO2 substrate has also been removed from the superstructures chemically to give hollow nano-structures. The successful extension of the work to the production of epitaxial rutile SnO2 NSFS indicates that this may be the basis for a much more general approach for the growth of metal oxide nanostructures.
[Small, 2010, 6(9), 988-992]
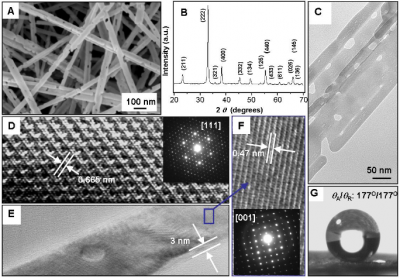
The construction of nanoporous membranes is of great technological importance for various applications, including catalyst supports, filters for biomolecule purification, environmental remediation and seawater desalination. A major challenge is the scalable fabrication of membranes with the desirable combination of good thermal stability, high selectivity and excellent recyclability. Here we present a self-assembly method for constructing thermally stable, free-standing nanowire membranes that exhibit controlled wetting behaviour ranging from superhydrophilic to superhydrophobic. These membranes can selectively absorb oils up to 20 times the material's weight in preference to water, through a combination of superhydrophobicity and capillary action. Moreover, the nanowires that form the membrane structure can be re-suspended in solutions and subsequently re-form the original paper-like morphology over many cycles. Our results suggest an innovative material that should find practical applications in the removal of organics, particularly in the field of oil spill cleanup.
[Nature Nanotechnology, 2008, 3, 332-336]
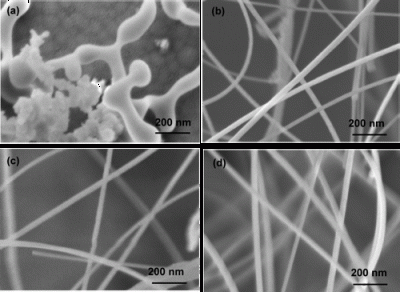
Silicon nanowires (SiNWs) have been fabricated by chemical vapor deposition at ambient pressure using SiCl4 as a silicon source and mesophase carbon microbead powder as a substrate without any templates and/or metal catalysts. The SiNWs have a crystalline core with a very thin amorphous SiOx sheath. The obtained SiNWs are homogeneous with average diameters below 50 nm and lengths up to micrometers. Temperature and time effects on the growth of SiNWs were systematically studied. Higher reaction temperatures and longer reaction times resulted in larger diameters and higher yields of SiNWs. SiNWs with a better crystallinity can be obtained at higher temperatures and longer reaction times. The obtained SiNWs were characterized by field-emission scanning electron microscopy, X-ray diffraction, Raman spectroscopy, and transmission electron microscopy.
[J. Phys. Chem. B, 2005, 109(8), 3291–3297]
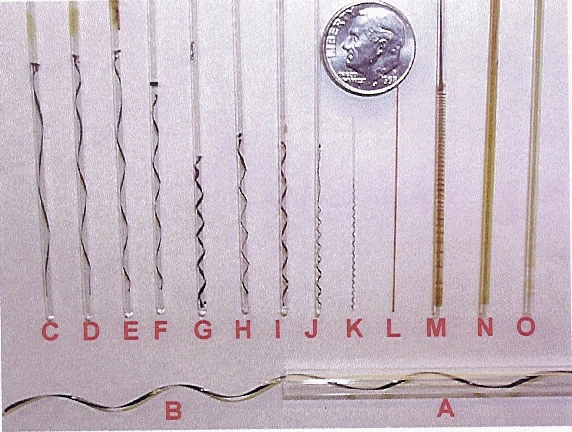
Attempts to generate films, wires, helices, rings and regular patterns out of inorganic materials have been going on for the past 100 years. We show here that stable helices of porous manganese oxide materials can be formed spontaneously from uniform sols and that they are excellent semiconductors. These inorganic helices contain micropores, can be converted into other structures, and their composition can be varied.
[Nature, 2000, 405, 38]